Application Notes
1. Time-dependent changes in hydrogen-terminated silicon
Purpose and Experiment
We investigated how silicon wafers terminated with hydrogen on their surfaces via HF treatment change over time. We desorbed the hydrogen adsorbed on the surface and performed evaluations.
The experiment and evaluation were conducted using the following procedure.
1. Prepare the hydrogen-terminated Si chips to be used in the experiment.
2. After sample preparation, perform temperature-programmed desorption experiments (desorption rate = 60K/sec) on Si chips after 2 days, 7 days, 14 days, and 28 days.
3. Compare the desorption signals for the desorbed hydrogen.
![]()
Results
As shown in the figure below, surface hydrogen decreases over time.
Further tracking experiments are needed to determine the extent of this decrease.
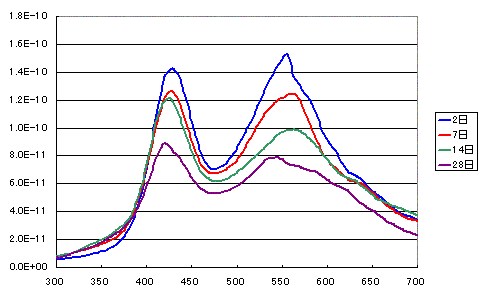
The decrease in surface hydrogen is thought to occur because oxidation progresses, generating hydroxyl groups on the surface.
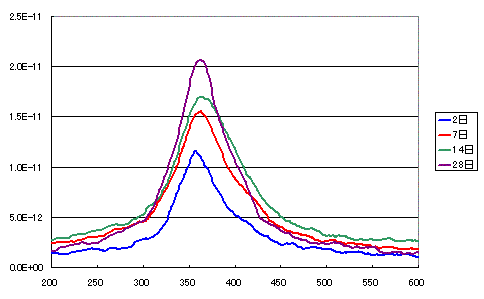
The following figure shows surface hydrogen and hydroxyl groups combining to form water, which then desorbs.
As expected, the amount of water increases over time.
![]()
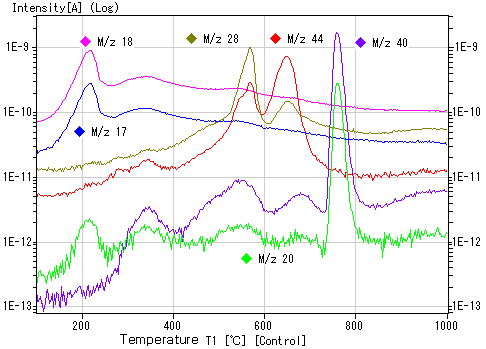
2. Hydrogen Ion Implantation Sample Data
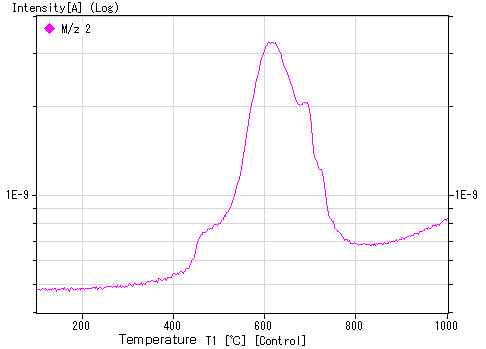
Example of hydrogen ion-implanted sample: 1E16 ions/cm²
Example of hydrogen ion implantation sample: 1E17 ions/cm²
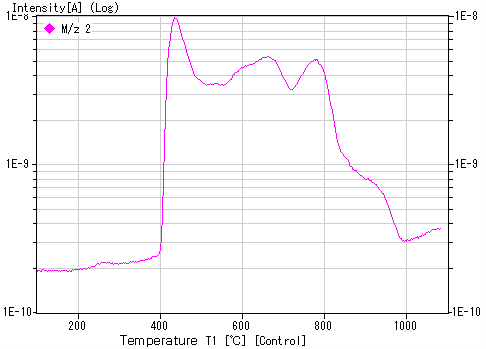
Note: The temperature at which the peak appears depends on various conditions, so slight variations may occur between devices.
![]()
3. Reproducibility Data for Hydrogen Ion Implantation Samples
Example of hydrogen ion implantation sample: 1E16 ions/cm²
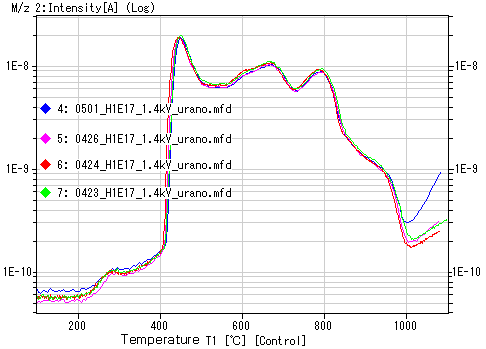
Note: The temperature at which the peak appears depends on various conditions, so slight variations may occur between devices.
![]()
4. Data for Calcium Oxalate-Coated Argon Ion Implantation Samples
Example of calcium oxalate-coated argon ion implantation sample
Note: The temperature at which the peak appears depends on various conditions, so slight variations may occur between devices.
![]()
5. Hydrogen Desorption (TDS) in SiNx Thin Films using ESCO-TDS1200II IR
Using an infrared-heating TDS system (Model: ESCO-TDS1200II IR), we evaluated hydrogen desorption in SiNx thin films. Three H2 desorption peaks were observed at 450 °C, 570 °C, and 980 °C. The low-temperature peaks are consistent with surface-origin species on the native oxide (H2O or H), whereas the high-temperature peak reflects bulk hydrogen (N–H and Si–H). These results provide actionable guidance for pre-clean, deposition, and annealing optimization.
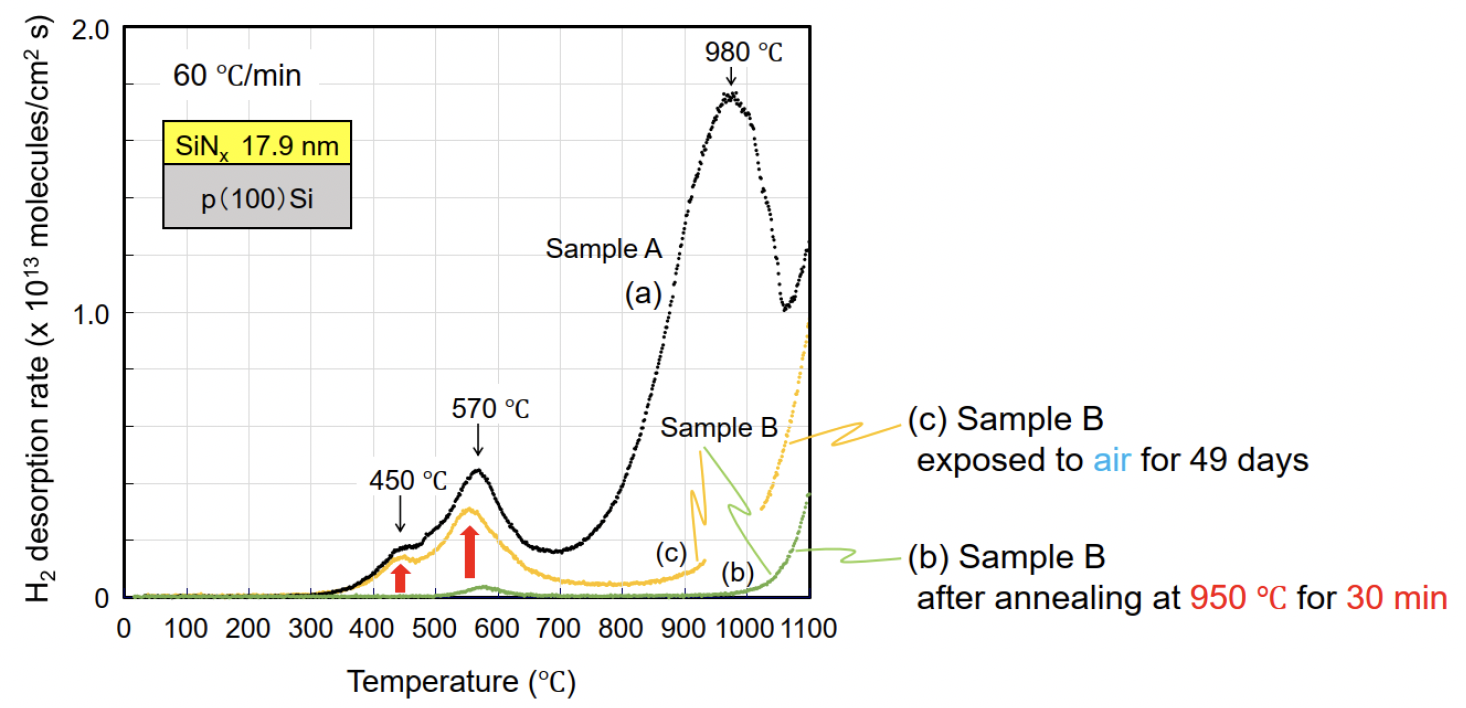
Fig. TDS spectra of H2 molecules obtained from samples A and B.
Low-temperature regime (450 °C and 570 °C) — Surface-origin desorption
High-temperature regime (around 980 °C) — Bulk-bonded hydrogen in SiNₓ
For additional technical details, please visit the Technical Note below.
⇒AppricationNote_TDS_SiNx_EN_20241114v3.pdf ※A PDF file (365kb) will open.
![]()
6. Hydrogen Desorption under an Anneal-like Temperature Profile in SiNₓ Thin Films using ESCO-TDS1200II IR
Using the ESCO-TDS1200II IR, we measured hydrogen release from SiNₓ thin films under a program similar to a common annealing furnace: a 10 °C/min ramp to 950 °C, followed by a 30-minute isothermal hold. Three H₂ peaks appear during heating, and the desorption rate falls steadily during the hold; about 65% of the total release occurs during the ramp.
Fig. 1 During the heating segment, three well-resolved H₂-desorption peaks emerged as the temperature increased.
During the isothermal segment at 950 °C, the H₂-desorption rate decayed smoothly and monotonically with time.
For additional technical details, please visit the Technical Note below
⇒AppricationNote _TDS_SiNx_EN_20251215v2.pdf ※A PDF file (320kb) will open.
![]()
7. Hydrogen Desorption Characteristics of Hydrogen-Charged Low-Carbon Mild Steel Sheets (Low-Temperature Heating Desorption)
⇒Hydrogen Desorption Characteristics of Hydrogen-Charged Low-Carbon Mild Steel Sheets ※A PDF file (170kb) will open.
Contact Us
If you have any questions or concerns about our products,
please feel free to contact us using the inquiry form below.
